Essential oils, chemical messengers, are used by aromatic plants to interact with their environment. These oils help to ward off disease and parasites, and also play a protective role against the sun’s rays. They also play an important role in the reproduction and dispersal of plant species, attracting pollinating insects.
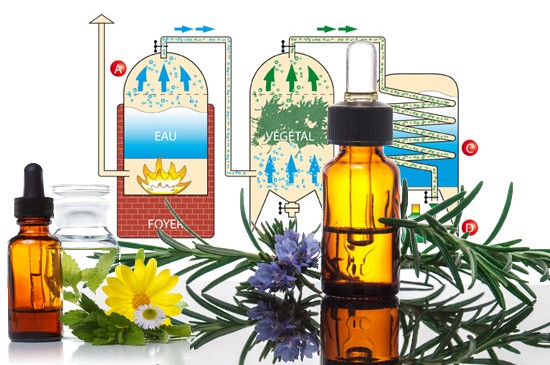
Steam distillation of an essential oil
Steam distillation is the main method for extracting essential oils, and the only one authorised by the European Pharmacopoeia, along with cold expression for citrus peel oils. Invented by the Pharaohs and improved by the Arabs, it uses a still, generally made of copper or stainless steel, with a sieve to prevent the plants coming into direct contact with the water.
As the water vapour passes through the plants, it carries away the micro-droplets of essential oil. Cooled in a coil, this vapour condenses, separating the essential oil from the water using a Florentine vase, due to their difference in density.
The technique involves heating the water until it boils, creating vapour which extracts the volatile components from the plants. These vapours rise in a cooler, cooled to between 15°C and 18°C to avoid the formation of crystals or insufficient condensation. As they condense, the vapours dri p into a container, forming the distillate, a mixture of essential oil and water. This mixture is then separated by decantation, using an organic solvent such as diethyl ether. The essential oil is mixed with the ether, dried over anhydrous sodium sulphate and then stored in opaque glass bottles at 4°C.
This method avoids direct contact of the oils with water, preventing degradation and preserving the quality of the oil. It is ideal for extracting essential oils from the surface of plants. Steam distillation takes longer and requires more steam for non-surface oils, but it allows faster, more delicate extraction, enriching the top notes, which are essential in industry and perfumery. For aromatherapy, distillation is prolonged to recover all the volatile components.
Hydrodistillation
Hydrodistillation is a simple method of extracting essential oils. It involves immersing the plant material in water, then bringing the mixture to the boil under atmospheric pressure. The heat releases the fragrant molecules from the plant cells. The boiling water solubilises part of the essential oil, which vaporises with the water vapour to form an azeotropic mixture. In this way, essential oils, which normally boil at between 200 and 300°C, evaporate at a temperature close to that of water. Once cooled, the water and essential oils separate into two phases.
However, direct contact with water can cause hydrolysis. The heat of hydrodistillation can chemically modify and degrade the heat-sensitive components of essential oils. The recovered oil therefore differs from the original essence, especially if distillation lasts a long time (3 hours). Hydrodistillation can be optimised by installing an electric agitator in the mixture, thereby reducing distillation time and energy consumption. This technique can also be used to extract oils from plants that are difficult to distil, such as wood, roots or bulbs.
However, hydrodistillation does have its limitations. Prolonged heating can damage certain plants and degrade aromatic molecules. Water, acidity and temperature can induce chemical reactions such as ester hydrolysis, rearrangement or oxidation. These reactions explain the wide variations in the composition of essential oils obtained by this method.
Cold expression
The cold expression extraction process is undoubtedly the simplest, but unfortunately also the most limited. This operation consists of using various processes to burst the ” pockets ” on the surface of the rind of these fruits, containing the essential oil (bergamot, orange, lemon, grapefruit, mandarin, etc.) essence “This is because the extraction method does not alter the plant product. Calabria and Sicily are responsible for worldwide production.
The cold expression technique is usually reserved for varieties of fruit or plants such as citrus fruits (oranges, lemons, mandarins, etc.). The essential oils of these fruits are contained in the small glands of their peel (zests).
This method is carried out without heating: it involves subjecting the plant substance to high pressure using a hydraulic press. This is done using sophisticated machinery. Prior to this mechanisation, cold extraction methods had long been traditional.
Cold pressing is a technique that originated in Sicily and is now used in all citrus-growing countries. In the past, it was done by hand using a process known as “sponging”: the fresh lemon was cut in half, the pulp was removed and the wet peel was left to rest for around ten hours. The peel was then pressed several times to extract the essence, against an assembly of sponges fixed in a vase. The pressure was checked by rotating the hand using a specially designed stick. After decanting, it was possible to recover (provided you had the necessary dexterity, or after a good apprenticeship) a very fine essence from the earthen vase by wringing out the sponges.
Enfleurage
Enfleurage, or saturation maceration, is an ancient technique that is rarely used today. Suitable for plants whose aroma cannot withstand the heat of distillation, it consists of interposing fragile plant substances between two layers of fat, which are renewed until saturation is reached. The excess fat is then removed to obtain an absolute essence, an essential oil of high olfactory quality.
Some plants, particularly flowers such as jasmine or rose, contain few aromatic molecules, which are difficult to extract by distillation. Extraction into absolutes more faithfully recreates their fragrance. Enfleurage, an ancient method used mainly in perfumery, involves placing the flowers in fat. Once saturated, the fat is washed with alcohol to extract the aromas, giving the absolute. This method, which is costly and uses animal fat, has been largely replaced by solvent extraction.
There are two types of enfleurage: cold and hot. Cold enfleurage, although expensive, treats fragile flowers. The odourless fat absorbs the odour of the flowers for three months. The perfumed fat is then treated with alcohol, and the absolute is obtained after the alcohol has been removed by distillation. Industrially, this technique was abandoned around 1930.
Hot enfleurage, practised since Antiquity, involves melting fat and mixing in flowers. The mixture is stirred and the flowers are regularly replaced. After saturation, the perfumed fat is treated in the same way as cold enfleurage to obtain a perfumed ointment.
Extracting an essential oil using solvents
Today, the solvent extraction technique replaces enfleurage to obtain absolutes prized in perfumery for their pure, powerful scent. This method involves macerating plants in volatile solvents such as hexane. Once the solvent has evaporated, a highly aromatic concrete is obtained. This concrete is diluted in ethyl alcohol, filtered and then concentrated by distillation under reduced pressure to remove the alcohol, producing the absolute. Supercritical CO2 extraction, used for barks, seeds or spices, is also popular in the food flavouring and perfume industries.
In solvent extraction, the flowers are macerated in a volatile hydrocarbon solvent, such as hexane. The solvent then evaporates, leaving a concrete containing aromatic compounds, waxes and oils from the plant. The concrete is macerated in alcohol to reduce the wax and obtain the absolute. The alcoholic solution is homogenised, refrigerated to precipitate the waxes, then the alcohol is evaporated to obtain the absolute.
The most common solvents are hexane, cyclohexane and ethanol. Extraction is carried out using a Soxhlet apparatus. Depending on the solvent, various types of extract are obtained, including hydrolysates, alcoholates, tinctures, resinoids and concretes.
Conventional solvent extraction involves placing the plant material in an extractor with a volatile solvent. After successive washings, the solvent loaded with aromatic molecules is distilled at atmospheric pressure. Although more expensive, this technique generally offers higher yields than distillation and avoids hydrolysis by steam. New techniques, such as microwave-assisted extraction and supercritical CO2 extraction, have been developed to improve efficiency and environmental safety.
Microwave extraction
Microwaves are used in two types of extraction:
- vMHD (vacuum microwave hydrodistillation)
- eSSAM (microwave-assisted solvent-free extraction).
In VMHD, only the water in the plant is involved in the extraction. The water molecules absorb the waves, generating heat that bursts the plant’s cellular structure, starting the hydrodistillation process. The vapour extracts the contents of the cells, then condenses into a liquid.
This technique releases the cell structure more quickly, saving time and water, and leaves no trace of solvent in the essential oil. ESSAM is used to extract fresh plant material at atmospheric pressure without water or solvents. The plants are placed in a reactor in a microwave oven. Internal heating of the plant’s intrinsic water expands its cells, causing azeotropic distillation of a water/essential oil mixture. An external refrigeration system condenses the distillate, which is then directed into the Clevenger apparatus for phase separation.
Compared with traditional distillation, this solvent-free microwave extraction method produces an essential oil of similar quality in less time. It favours the extraction of oxygenated compounds, which are more fragrant than monoterpenes. The technique reduces distillation time and increases yield, but has not yet been developed industrially. It offers a number of advantages: green technology, energy and time savings, low initial investment and minimisation of thermal and hydrolytic degradation.
SFME (Solvent Free Microwave Extraction) combines microwave heating and dry distillation techniques. Without the addition of water or solvent, the internal heating of the plant water causes the glands and oleiferous vessels to rupture, releasing the essential oil, which evaporates with the plant water. This technique is faster, saves energy and produces essential oils that are richer in oxygenated compounds.
Supercritical CO2 extraction
Supercritical CO2 extraction, similar to solvent extraction, uses CO2 as a non-harmful solvent, leaving no traces in the essential oil obtained. To reach the supercritical state, an element is subjected to high pressure or temperature. CO2 easily reaches this state: it simply needs to be heated to 31°C or pressurised to 74 bars. Other supercritical fluids can also be used.
This process involves placing crushed plants in an extractor, then exposing them to supercritical CO2, compressed and heated to 40°C. The essential oil dissolves and the CO2 becomes gaseous again, separating easily from the oil. The oil remains pure and true to the plant’s original substance. However, this method is not widely used due to the high cost of the equipment.
Extraction with supercritical CO2 is distinguished by its solvent: CO2 in the supercritical phase, which is neither liquid nor gaseous. It is an excellent solvent in the supercritical state, but not very effective in the gaseous state. Its advantages include its inert, non-toxic and inexpensive nature, the use of low temperatures, and the ease with which the solvent can be separated at the end of the cycle. This technique, which is more complete and less degrading than water vapour, offers new olfactory notes. However, the cost of industrial installation remains high.
Sources
- https://www.doc-developpement-durable.org/file/Fabrications-Objets-Outils-Produits/Huiles-essentielles/FICHES_PLANTES&HUILES/Huile-essentielle_Wikipedia_FR.pdf
- https://agrobiologia.net/online/wp-content/uploads/2020/01/18-1653-1659_-BOUKHATEM-et-al_.pdf
- https://fr.wikipedia.org/wiki/Enfleurage
- https://dumas.ccsd.cnrs.fr/dumas-01919474/document
- https://dumas.ccsd.cnrs.fr/dumas-01515314/document
- https://www.ummto.dz/dspace/bitstream/handle/ummto/1568/THESE.pdf?sequence=1&isAllowed=y